- Details
- Tuesday, 23 May 2017

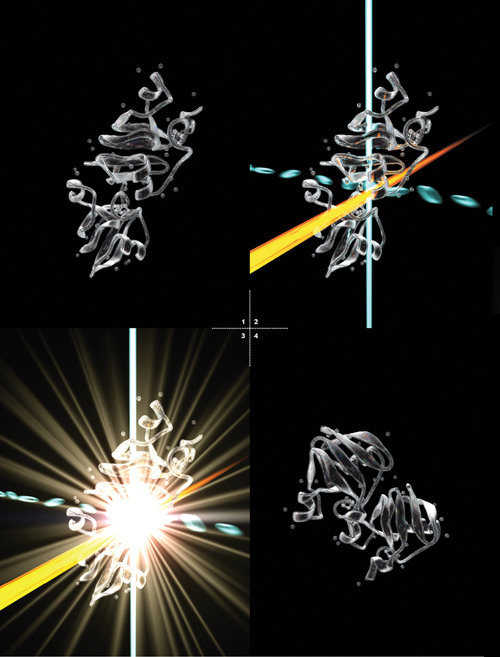
Burrowed deep under the foothills near Palo Alto, Calif., scientists scurried through an underground laboratory, making final preparations for a series of explosions. THEIR PLAN: blow up tiny crystals of proteins that could reveal one of nature's best-kept secrets—how plant photosynthesis turns light into chemical energy. The potential payoff: a step toward unlimited clean power.
It was December 2009, and a sleep-deprived team of researchers and students at SLAC National Accelerator Laboratory had been working nonstop for days to set up this experiment at the world's most powerful x-ray laser, the Linac Coherent Light Source (LCLS), which accelerates electrons to nearly the speed of light. One group feverishly adjusted injectors that would shoot crystals of proteins into the x-ray beam. Another locked and loaded the injector with fresh crystals of a protein complex called photosystem I, which is key to photosynthesis.
At the end of the two-mile accelerator tunnel, the crystals began their march into the intense laser light. But before each of them exploded, its snapshot was taken with a newly developed scientific technique. Today that method promises to reshape our understanding of biology on the tiniest scale because we can now assemble a rapid sequence of such images—shot in femtoseconds, or millionths of a billionth of a second—into movies.
Physicist Richard Feynman once said, “Everything that living things do can be understood in terms of the jigglings and wigglings of atoms.” But never before have we been able to directly see the wiggling of atoms and molecules within living things at this speed. Our method, called serial femtosecond crystallography (SFX), lets us watch high-speed molecular dances that determine how medicines affect diseased cells and how chemical reactions convert energy to different forms. See full article here
