- Details
- Monday, 10 September 2018

Antibiotic resistance is an imminent threat to human health. Tuberculosis is the number one killer among infectious diseases. Bacteria that cause tuberculosis are difficult to treat because they are often antibiotic resistant.
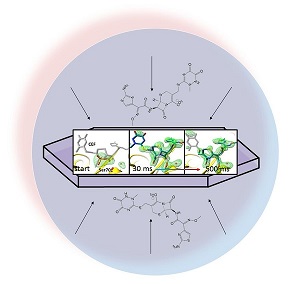
One of the causes of antibiotic resistance are β-lactamases which broadly inactivate β-lactam antibiotics such as penicillin and cephalosporin compounds. In experiments led by BioXFEL scientists the process of cephalosporin inactivation has been demonstrated in real-time and ambient temperatures using mix-and-inject serial crystallography (MISC) at the Linac Coherent Light Source (LCLS). In these experiments, a third generation cephalosporin, ceftriaxone (CEF), was mixed with Mycobacterium tuberculosis β-lactamase (BlaC) microcrystals, and the reaction was monitored with millisecond time-resolution at near atomic resolution. Structures of the reactions were obtained after 30 ms, 100 ms, 500 ms and 2 s. Already 30 ms after mixing, full occupancy of the CEF was achieved in the active site. The β-lactam ring opening with concomitant cleavage of a release group from the CEF happened at 500 ms. At 2 s, enough product accumulated to slow down the reaction. A compatible kinetic mechanism was developed that explained the experiment data. This is the first time that an enzymatically catalyzed reaction could be characterized at an X-ray free electron laser. MISC as developed by BioXFEL becomes a versatile and general method to structurally investigate reactions of bio-medically important biological macromolecules in real time.
The results of the experiments were published on May 1 2018 in BMC Biology.
https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-018-0524-5
