Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- Structural biology is solved -- now what?
- XFEL Pulses Demonstrate How Plants Perceive Light
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
- Details
- Wednesday, 28 August 2024

A group of BioXFEL researchers brought single particle cryoEM structure determination to BioXFEL. The research was spearheaded by Tek Malla, a BioXFEL graduate in the Schmidt lab.
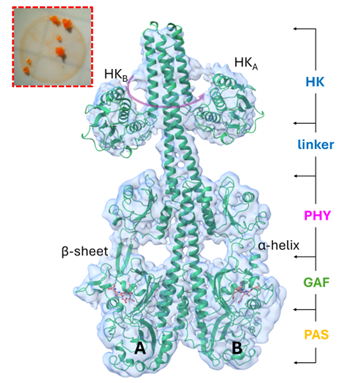
Using samples of a bacterial phytochrome prepared in the Stojkovic lab at Northeastern Illinois University, cryo-EM grids were prepared, and single particle cryo-EM experiments were conducted by scientists at the New York Structural Biology Center. The researchers investigated bacteriophytochrome 2 (SaBphP2) from the myxobacterium Stigmatella aurantiaca. Bacteriophytochromes are enzyme photoreceptors that consist of a photosensory module with three domains (PAS, GAF and PHY) and an enzymatic domain which is typically a histidine kinase (HK). Light is absorbed by a central chromophore located at the boundary of the PAS and GAF domains. The chromophore is biliverdin in bacterial phytochromes. By shining red light with 660 nm wavelength on SaBphP2 its conformation shifts from a red-absorbing Pr state to a far-red absorbing Pfr state. The Pr to Pfr transition is accompanied by large structural changes of the phytochrome. The transition is driven by the Z to E isomerization of a central biliverdin (BV) chromophore. It can be reversed by far-red light (λ=750 nm), and the Pr state with the chromophore in the Z configuration is recovered. Using single particle cryo-EM the structures of the Pr and Pfr state of the full length SaBphP2 that includes the enzymatic domain were determined. Large domain rotations were observed. Most importantly, the HK lobes rotate by about 70o.
This constitutes a potential signaling mechanism involving the binding and dissociation of a more downstream response regulator. Surprisingly, and most importantly, a hybrid form with one subunit in the Pr and the other in the Pfr state was observed. The existence of a Pr/Pfr heterodimer has been postulated, but it was never observed except in mutants of a phytochrome from a marine organism. The occurrence of the Pr/Pfr heterodimer not only in the full-length phytochrome but also in a shorter construct that lacks the linker and the enzymatic domain shows that heterodimer formation does not depend on the linker region.
The results suggest that the heterodimer plays an important role in the signal transduction in myxobacteria and most likely in all other organisms carrying phytochromes. The research has been published on Aug 9 2024 in Science Advances (DOI: 10.1126/sciadv.adq0653).





