Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- XFEL Pulses Demonstrate How Plants Perceive Light
- Structural biology is solved -- now what?
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
- Details
- Thursday, 04 December 2014

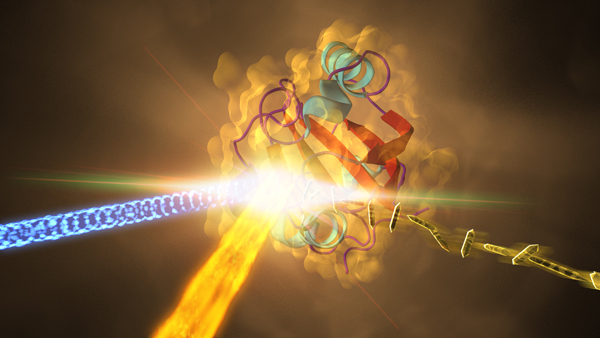
BioXFEL researchers have captured the highest-resolution protein snapshots ever taken with an X-ray laser, revealing how a key protein in a photosynthetic bacterium changes shape when hit by light.
Sharpest Protein Images Bring Atomic-scale Biology Within Reach
Human biology is a massive collection of chemical reactions, from the intricate signaling network that powers our brain activity to the body’s immune response to viruses and the way our eyes adjust to sunlight. All involve proteins, known as the molecules of life; and scientists have been steadily moving toward their ultimate goal of following these life-essential reactions step by step in real time, at the scale of atoms and electrons.
"These results establish that we can use this same method with all kinds of biological molecules, including medically and pharmaceutically important proteins" said Marius Schmidt, a biophysicist at the University of Wisconsin-Milwaukee who led the experiment at the Department of Energy’s SLAC National Accelerator Laboratory. There is particular interest in exploring the fastest steps of chemical reactions driven by enzymes – proteins that act as the body's natural catalysts, he said. "We are on the verge of opening up a whole new unexplored territory in biology, where we can study small but important reactions at ultrafast timescales,” Schmidt said.
The results, detailed in the Dec. 5 issue of Science, have exciting implications for research on some of the most pressing challenges in life sciences, which include understanding biology at its smallest scale and discovering molecular targets for drug design.
X-ray Laser Proven as Tool to Track Life’s Chemistry
(reprinted from SLAC News Center)
A New Way to Study Shape-shifting Proteins
The experiment took place at SLAC’s Linac Coherent Light Source (LCLS), a DOE Office of Science User Facility. LCLS’s X-ray laser pulses, which are about a billion times brighter than X-rays from synchrotrons, allowed researchers to see new atomic-scale details of how the bacterial protein changes within millionths of a second after it’s exposed to light.
"This experiment marks the first time LCLS has been used to directly observe a protein’s structural change as it happens. It opens the door to reaching even faster time scales," said Sebastien Boutet, a SLAC staff scientist who oversees the experimental station used in the study. LCLS's pulses, measured in quadrillionths of a second, work like a super-speed camera to record ultrafast changes, and snapshots taken at different points in time can be compiled into detailed movies.
The protein the researchers studied, found in purple bacteria and known as PYP for "photoactive yellow protein," functions much like a bacterial eye in sensing certain wavelengths of light. The mechanism is very similar to that of other receptors in biology, including receptors in the human eye. "Though the chemicals are different, it's the same kind of reaction," said Schmidt, who has studied PYP since 2001. Proving the technique works with a model protein like PYP sets the stage to study more complex and biologically important molecules at LCLS, he said.
Chemistry on the Fly
In the LCLS experiment, researchers prepared crystallized samples of the protein, and exposed the needle-shaped crystals, each about 2 millionths of a meter long, to optical laser light before jetting them into the LCLS X-ray beam.
The X-rays produced patterns of light as they struck the crystals, which are used to reconstruct the 3-D structures of the proteins. Researchers compared the structures of the light-exposed proteins to those of proteins that had been jetted in the dark to identify light-induced structural changes.
"In the future we plan to study all sorts of enzymes and other proteins using this same technique," Schmidt said. "This study shows that the molecular details of life’s chemistry can be followed using X-ray laser crystallography, which puts some of biology’s most sought-after goals within reach.
Citation: Jason Tenboer, Shibom Basu, et al., Science, 5 December 2014, ...





