Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- XFEL Pulses Demonstrate How Plants Perceive Light
- Structural biology is solved -- now what?
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
- Details
- Tuesday, 16 February 2016
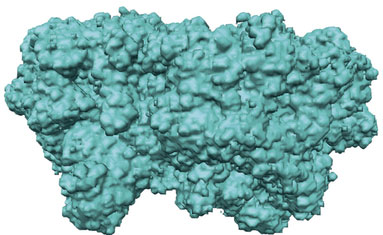
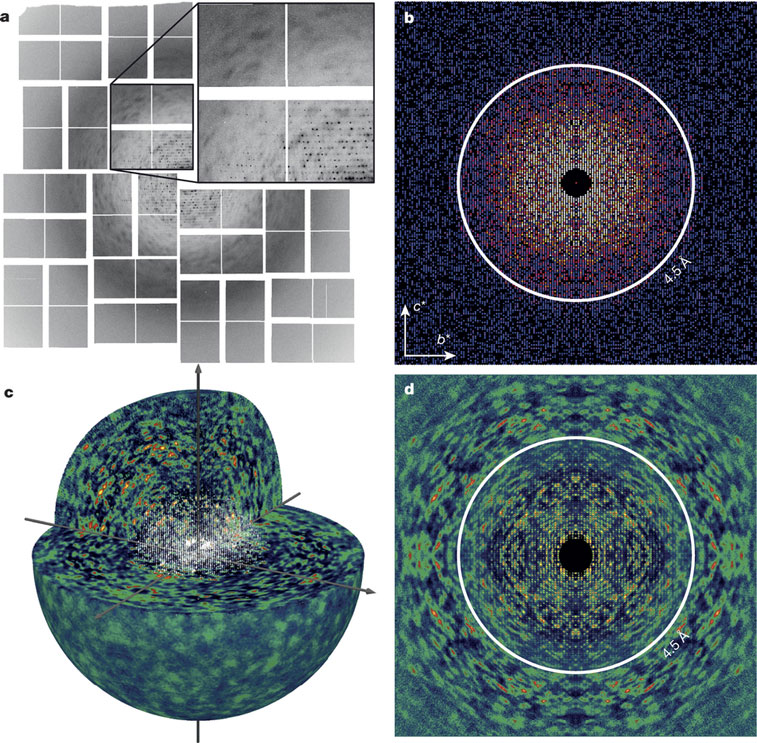 Protein structure determination by X-ray crystallography is often limited by lack of access to high-quality crystals that generate sufficiently detailed diffraction patterns. However, X-ray patterns usually also contain continuous diffraction, which is largely ignored but could in principle provide sufficient information to overcome this limitation.
Protein structure determination by X-ray crystallography is often limited by lack of access to high-quality crystals that generate sufficiently detailed diffraction patterns. However, X-ray patterns usually also contain continuous diffraction, which is largely ignored but could in principle provide sufficient information to overcome this limitation.
Kartik Ayyer and colleagues now show that the continuous diffraction arising from lattice disorder indeed enables structure determination. They use data collected from imperfect crystals of the protein complex photosystem II to obtain an image at 3.5 Å resolution. The method puts great value in commonly encountered imperfect crystals, and is expected to enable direct high-resolution structure determination for a range of macromolecular systems.
A new article published in Nature describing this new technique includes an international team of researchers led by BioXFEL collaborator Henry Chapman. To learn more click here.