Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- XFEL Pulses Demonstrate How Plants Perceive Light
- Structural biology is solved -- now what?
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
- Details
- Thursday, 06 April 2017

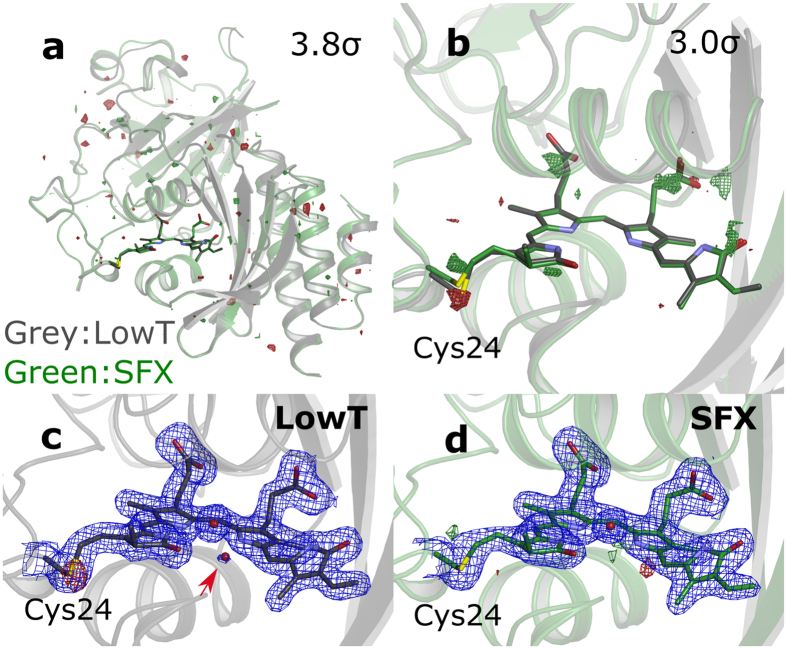
Phytochromes are a family of photoreceptors that control light responses of plants, fungi and bacteria. A sequence of structural changes, which is not yet fully understood, leads to activation of an output domain. Time-resolved serial femtosecond crystallography (SFX) can potentially shine light on these conformational changes. Here we report the room temperature crystal structure of the chromophore-binding domains of the Deinococcus radiodurans phytochrome at 2.1 Å resolution.
The structure was obtained by serial femtosecond X-ray crystallography from microcrystals at an X-ray free electron laser. We find overall good agreement compared to a crystal structure at 1.35 Å resolution derived from conventional crystallography at cryogenic temperatures, which we also report here. The thioether linkage between chromophore and protein is subject to positional ambiguity at the synchrotron, but is fully resolved with SFX. The study paves the way for time-resolved structural investigations of the phytochrome photocycle with time-resolved SFX.





