Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- XFEL Pulses Demonstrate How Plants Perceive Light
- Structural biology is solved -- now what?
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
News
- Details
- Tuesday, 14 March 2017
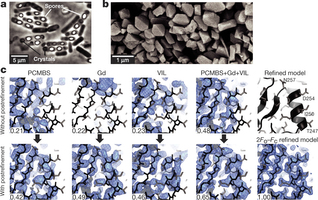
BinAB is a naturally occurring paracrystalline larvicide distributed worldwide to combat the devastating diseases borne by mosquitoes. These crystals are composed of homologous molecules, BinA and BinB, which play distinct roles in the multi-step intoxication process, transforming from harmless, robust crystals, to soluble protoxin heterodimers, to internalized mature toxin, and finally to toxic oligomeric pores.
- Details
- Thursday, 09 March 2017
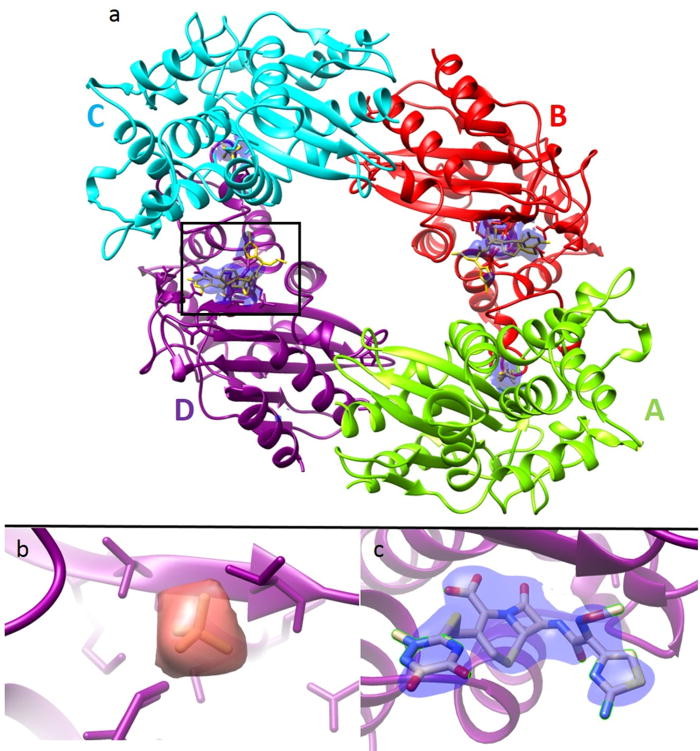
Mix-and-inject serial crystallography (MISC) is a technique designed to image enzyme catalyzed reactions in which small protein crystals are mixed with a substrate just prior to being probed by an X-ray pulse. This approach offers several advantages over flow cell studies. It provides (i) room temperature structures at near atomic resolution, (ii) time resolution ranging from microseconds to seconds, and (iii) convenient reaction initiation. It outruns radiation damage by using femtosecond X-ray pulses allowing damage and chemistry to be separated.
- Details
- Tuesday, 07 March 2017
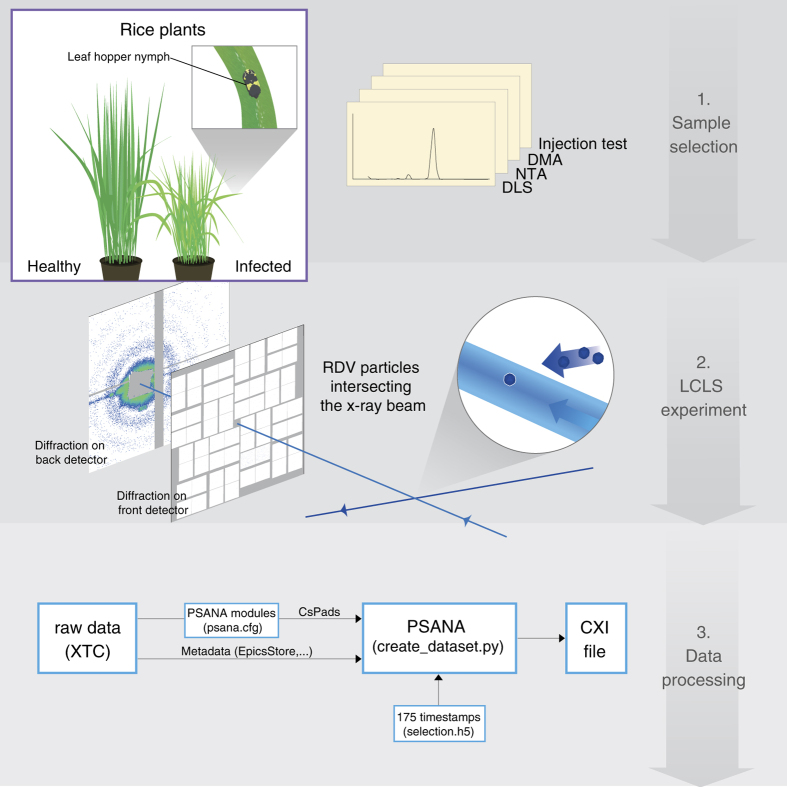
For several decades, X-ray crystallography has been the dominant technique to solve the three-dimensional (3D) structure of biological macromolecules at atomic resolution. Structures of proteins, protein complexes and the machinery of entire biological reaction pathways have been elucidated, leading to numerous breakthroughs in our understanding of molecular architecture and function. However, not every protein complex crystallizes, a necessary condition for investigation using these methods.
- Details
- Thursday, 02 March 2017
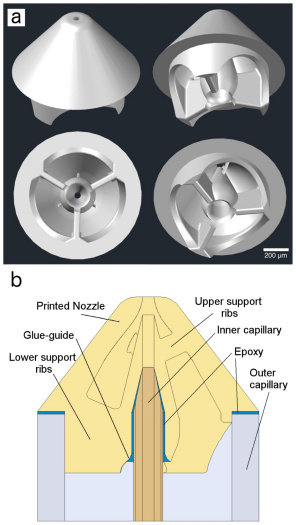
Reliable sample delivery is essential to biological imaging using X-ray Free Electron Lasers (XFELs). Continuous injection using the Gas Dynamic Virtual Nozzle (GDVN) has proven valuable, particularly for time-resolved studies. However, many important aspects of GDVN functionality have yet to be thoroughly understood and/or refined due to fabrication limitations. We report the application of 2-photon polymerization as a form of high-resolution 3D printing to fabricate high-fidelity GDVNs with submicron resolution.
- Details
- Tuesday, 28 February 2017
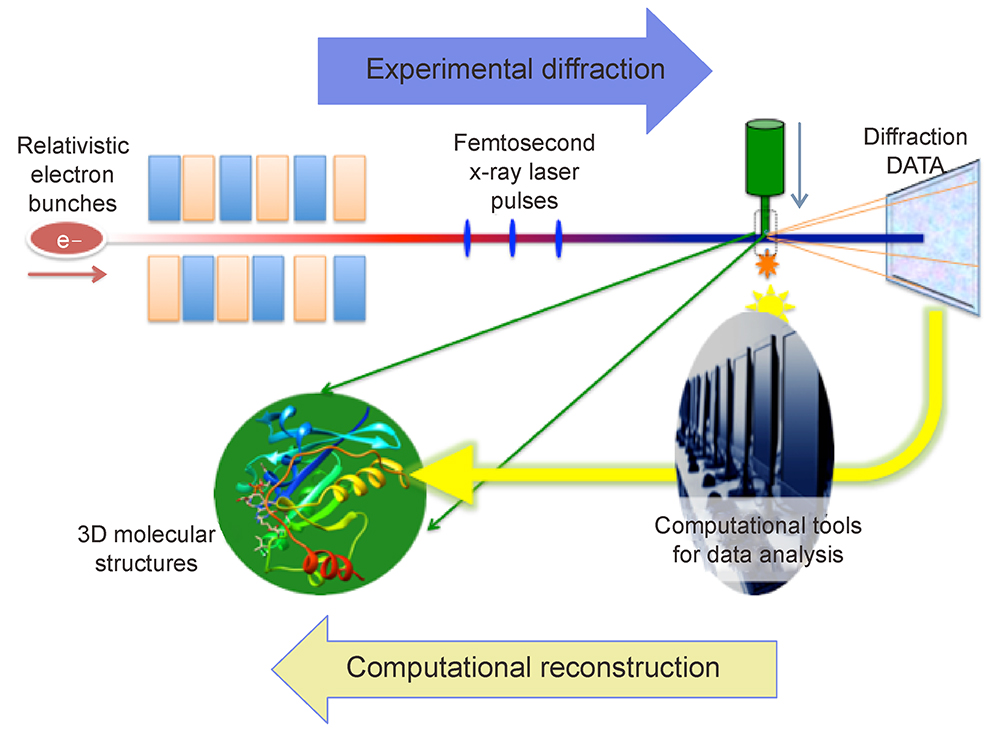
X-ray Free Electron Lasers (XFELs) have advanced research in structure biology, by exploiting their ultra-short and bright X-ray pulses. The resulting “diffraction before destruction” experimental approach allows data collection to outrun radiation damage, a crucial factor that has often limited resolution in the structure determination of biological molecules.
- Details
- Thursday, 23 February 2017
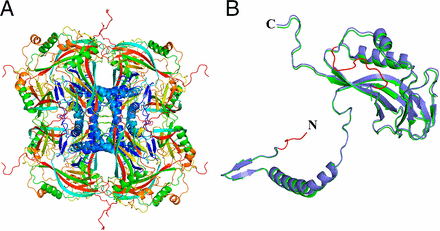
The room temperature structure of natively formed protein nanocrystals consisting of 9,000 unit cells has been solved to 2 Å resolution using an unattenuated X-ray free-electron laser (XFEL) beam, representing, by far, the smallest protein crystals used for protein structure determination by X-ray crystallography to date.
More Articles...
- Markelz receives $1.35 million to study molecules’ vibrations, opening new possibilities for an emerging field
- New way to discover structures of membrane proteins
- Nature article sheds light on new machine learning algorithms for protein structure predicition
- Measure by measure: X-rays show viral transformation
- Shanghai team develops 'world's brightest VUV free-electron laser'





