Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- XFEL Pulses Demonstrate How Plants Perceive Light
- Structural biology is solved -- now what?
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
News
- Details
- Thursday, 06 April 2017
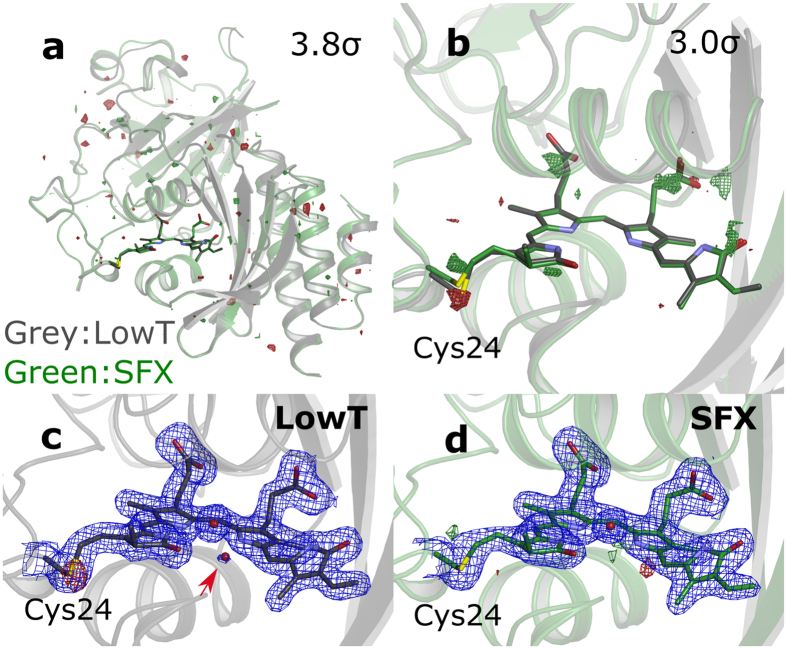
Phytochromes are a family of photoreceptors that control light responses of plants, fungi and bacteria. A sequence of structural changes, which is not yet fully understood, leads to activation of an output domain. Time-resolved serial femtosecond crystallography (SFX) can potentially shine light on these conformational changes. Here we report the room temperature crystal structure of the chromophore-binding domains of the Deinococcus radiodurans phytochrome at 2.1 Å resolution.
- Details
- Tuesday, 04 April 2017
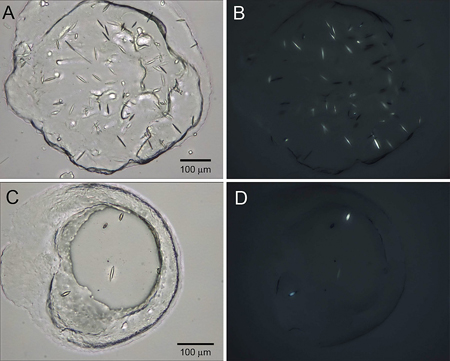
Membrane proteins perform critical functions in living cells related to signal transduction, transport and energy transformations, and, as such, are implicated in a multitude of malfunctions and diseases. However, a structural and functional understanding of membrane proteins is strongly lagging behind that of their soluble partners, mainly, due to difficulties associated with their solubilization and generation of diffraction quality crystals.
- Details
- Thursday, 30 March 2017

The BioXFEL Center is proud to announce the recipients of the Postdoctoral Research Award, Benjamin Stauch and Ahmad Hosseinizadeh. Dr. Stauch is a Postdoctoral Research Associate in the group of Prof. Vadim Cherezov at the Bridge Institute of the University of Southern California in Los Angeles. He received his Master’s degree in Bioinformatics from the University of Frankfurt (Germany) in 2007 and carried out his doctoral research within the EMBL International Ph.D. Programme at EMBL Heidelberg (Germany) and the University of Cambridge (UK) where he obtained a Ph.D. in Biology in 2013.
- Details
- Thursday, 30 March 2017
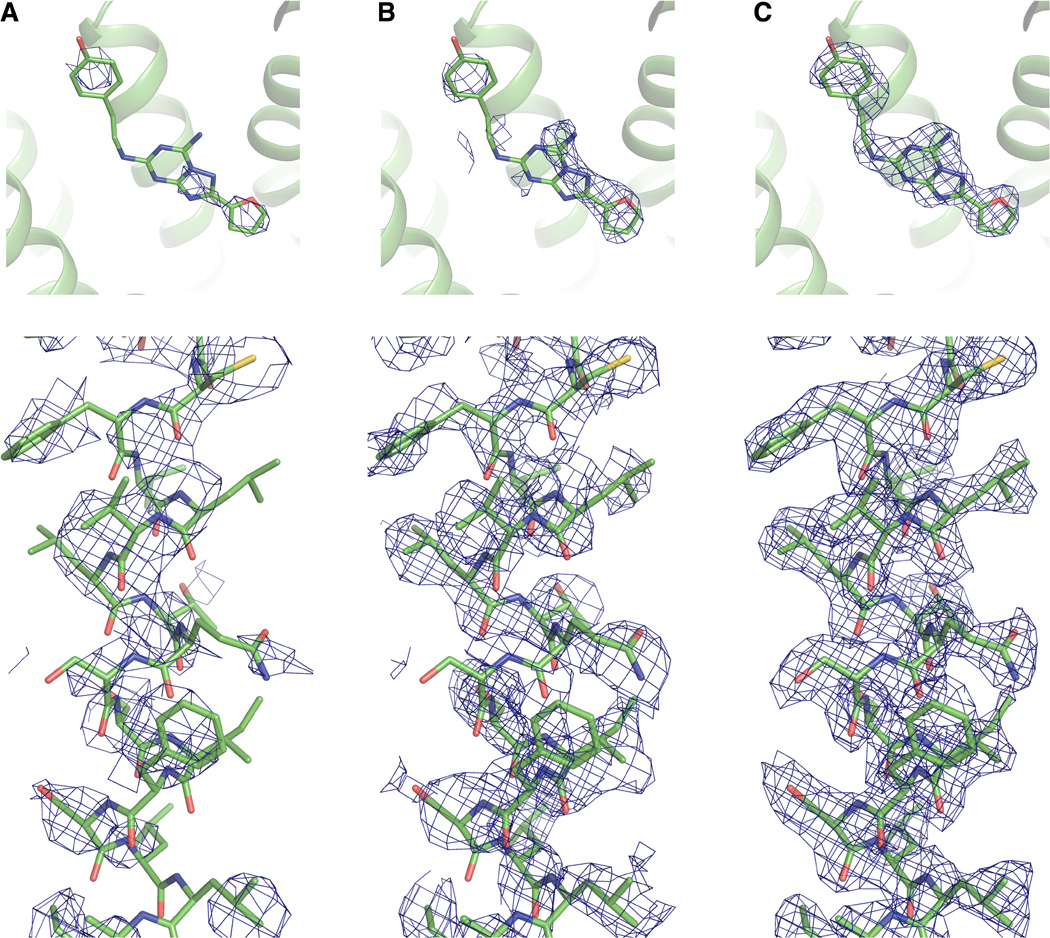
Serial femtosecond crystallography (SFX) takes advantage of extremely bright and ultrashort pulses produced by x-ray free-electron lasers (XFELs), allowing for the collection of high-resolution diffraction intensities from micrometer-sized crystals at room temperature with minimal radiation damage, using the principle of “diffraction-before-destruction.” However, de novo structure factor phase determination using XFELs has been difficult so far.
- Details
- Friday, 24 March 2017

Arizona State University is currently seeking a research specialist. This position in experimental biophysics would assist scientists in collecting experimental data at the Linac Coherent Light Source (LCLS) X-ray laser near Stanford University, as part of the BioXFEL project https://www.bioxfel.org/.
- Details
- Thursday, 16 March 2017
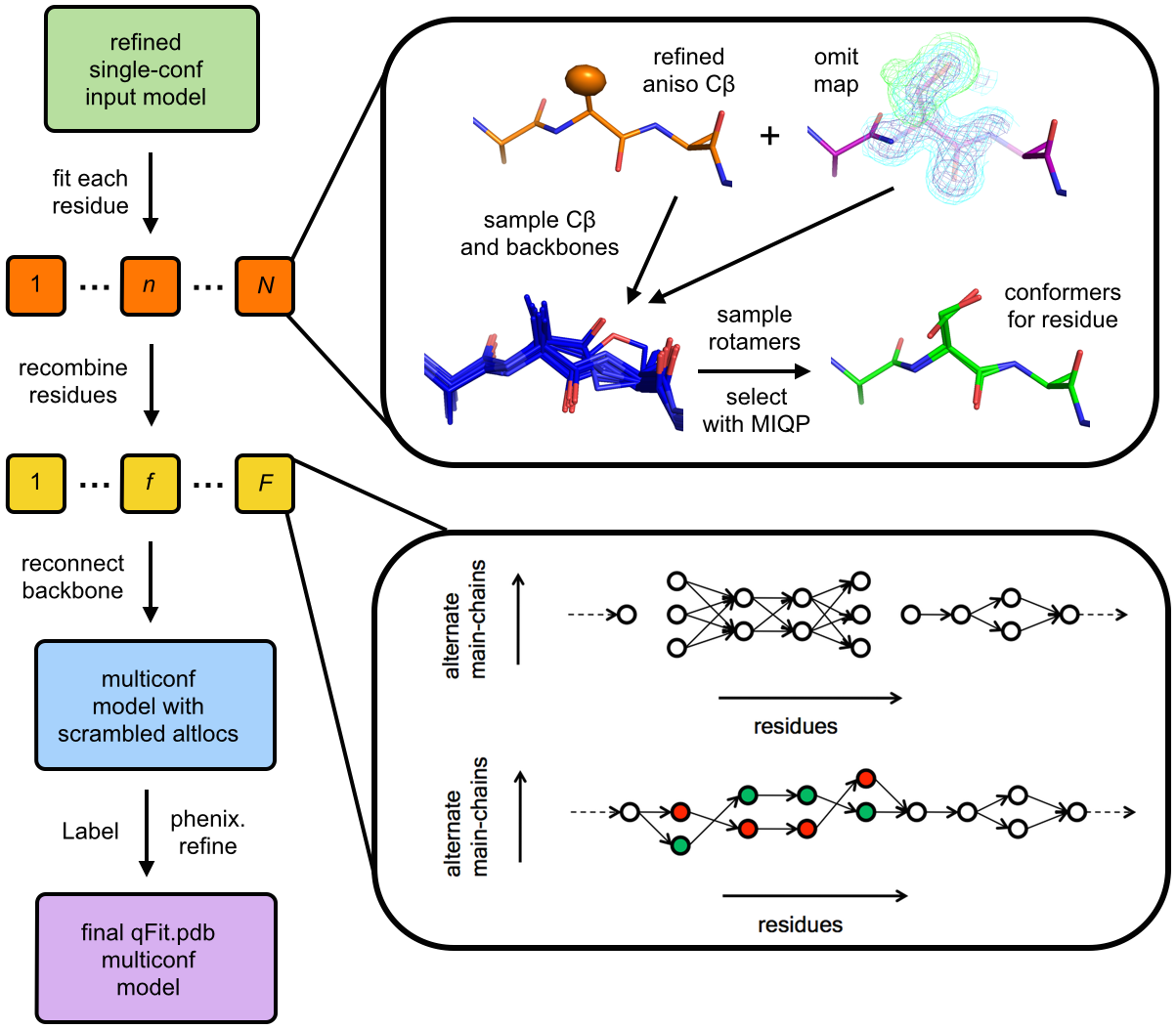
Describing the multiple conformations of proteins is important for understanding the relationship between molecular flexibility and function. However, most methods for interpreting data from X-ray crystallography focus on building a single structure of the protein, which limits the potential for biological insights. Here we introduce an improved algorithm for using crystallographic data to model these multiple conformations that addresses two previously overlooked types of protein backbone flexibility: peptide flips and glycine movements.
More Articles...
- De novo phasing with X-ray laser reveals mosquito larvicide BinAB structure
- Structural enzymology using X-ray free electron lasers
- Coherent diffraction of single Rice Dwarf virus particles using hard X-rays at the Linac Coherent Light Source
- Three-dimensional-printed gas dynamic virtual nozzles for x-ray laser sample delivery
- XFEL data analysis for structural biology





